predict the major product for the following reaction when drawing hydrogen atoms
17.10: Reactions of Phenols
-
- Last updated
- Save as PDF
- Page ID
- 36354
Objectives
After completing this section, you should be able to
- explain why phenols and phenoxide ions are very reactive towards electrophilic aromatic substitution (see Section 16.4 of the textbook).
- write an equation to illustrate the oxidation of a phenol or an arylamine to a quinone, and identify the reagents used to oxidize phenols.
- write an equation to illustrate the reduction of a quinone to a hydroquinone, and identify the reagents used to reduce quinones.
- describe, briefly, the biological importance of the redox properties of quinones.
Key Terms
Make certain that you can define, and use in context, the key terms below.
- hydroquinone
- quinone
- ubiquinone
Study Notes
"Quinone" is a term used to describe cyclohexadiendiones in general, and p‑benzoquinone in particular. In addition to benzene, other aromatic systems also give rise to quinones; for example, 1,4‑naphthoquinone
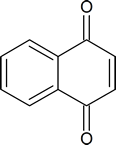
"Hydroquinones" are produced by the reduction of quinones according to the following half‑reaction:

"Ubiquinones" are naturally occurring quinones whose role is to transfer a pair of electrons from one substance to another in enzyme‑catalyzed reactions. Ubiquinones are also called coenzymes Q.

Electrophilic Aromatic Substitution Reactions
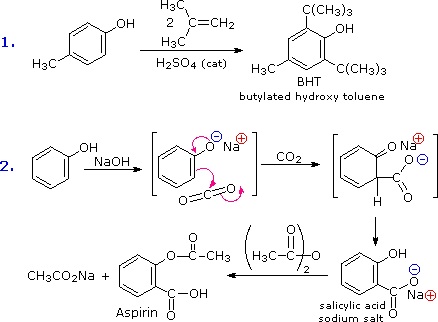
The facility with which the aromatic ring of phenols and phenol ethers undergoes electrophilic substitution has been noted. Two examples are shown in the following diagram. The first shows the Friedel-Crafts synthesis of the food preservative BHT from para-cresol. The second reaction is interesting in that it further demonstrates the delocalization of charge that occurs in the phenolate anion. Carbon dioxide is a weak electrophile and normally does not react with aromatic compounds; however, the negative charge concentration on the phenolate ring enables the carboxylation reaction shown in the second step. The sodium salt of salicylic acid is the major product, and the preference for ortho substitution may reflect the influence of the sodium cation. This is called the Kolbe-Schmidt reaction, and it has served in the preparation of aspirin, as the last step illustrates.
Oxidation of Phenols: Quinones
Phenols are rather easily oxidized despite the absence of a hydrogen atom on the hydroxyl bearing carbon. Among the colored products from the oxidation of phenol by chromic acid is the dicarbonyl compound para-benzoquinone (also known as 1,4-benzoquinone or simply quinone); an ortho isomer is also known. These compounds are easily reduced to their dihydroxybenzene analogs, and it is from these compounds that quinones are best prepared. Note that meta-quinones having similar structures do not exist. The redox equilibria between the dihydroxybenzenes hydroquinone and catechol and their quinone oxidation states are so facile that milder oxidants than chromate (Jones reagent) are generally preferred.
One such oxidant is Fremy's salt, shown on the right. Reducing agents other than stannous chloride (e.g. NaBH4) may be used for the reverse reaction. The position of the quinone-hydroquinone redox equilibrium is proportional to the square of the hydrogen ion concentration, as shown by the following half-reactions (electrons are colored blue). The electrode potential for this interconversion may therefore be used to measure the pH of solutions.
| Quinone + 2H(+) | 2e(–) –2e(–) | Hydroquinone |
|---|

Exercises
Exercise \(\PageIndex{1}\)
Add exercises text here.
- Answer
-
Add texts here. Do not delete this text first.
Exercise \(\PageIndex{1}\)
Add exercises text here.
- Answer
-
Add texts here. Do not delete this text first.
Questions
Q17.10.1
Predict the major product if the following reagents/reagents were used. No reaction is also a possible answer.

(a) 1 equivalent of PBr3 (b) 1 equivalent of SOCl2 (c) Dess–Martin periodinane (d) 3 equivalents of acetyl chloride and AlCl3 as a catalyst
(e) Heat and H2SO4 (assume the phenol does not act as a nucleophile in this case)
Q17.10.2
Predict the major product if the following reagents/conditions were used. No reaction is also a possible answer.

(a) 2 equivalents of RMgBr and H3O+ work-up (b) LiAlH4 and H3O+ work-up (c) NaBH4 and H3O+ work-up
Solutions
S17.10.1
(a)  (b)
(b)  (c)
(c)  (d)
(d)  (e)
(e) 
S17.10.2
(a)  (b)
(b)  (c) No reaction. NaBH4 is milder oxidant than LiAlH4. It typically only reduces ketones and aldehydes.
(c) No reaction. NaBH4 is milder oxidant than LiAlH4. It typically only reduces ketones and aldehydes.
predict the major product for the following reaction when drawing hydrogen atoms
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_%28McMurry%29/17:_Alcohols_and_Phenols/17.10:_Reactions_of_Phenols
Posted by: montgomerycourer1950.blogspot.com

0 Response to "predict the major product for the following reaction when drawing hydrogen atoms"
Post a Comment